WONDERFUL! Why Is Copper A Good Conductor
This high charge density that copper possesses is the responsible for the slow drift of velocity that occurs when electrical charges are. Copper is a good conductor of heat.

Gbc Gibraltar Broadcasting Corporation Electrical Cables Copper Wire Electricity
The invention of the telephone in 1876 created further demand for copper wire as an electrical conductor.

Why is copper a good conductor. Copper is a good conductor of heat. Copper has low resistivity and therefore is an excellent conductor. Copper is also easy to solder and wrap into wires so it is often used when a large amount of conductive material is required.
There are still some questions as to why certain metals are better conductors of heat and why certain materials have higher specific heats than others but right now it appears to be the shape of the atoms nucleus and how the Protons are arranged that are major factors in heat co. For a material to be a good conductor the electricity passed through it must be able to move the electrons. Copper is a good conductor of electricity because the valence electrons are free and repel each other so strongly that it causes the repulsion of other electrons.
Aluminium has 61 of the conductivity of copper. However apart from silver copper is the best. The electrons can move freely through the metal.
Why is copper a good conductor. Many common applications also rely on one or more beneficial properties such as the fact that it is a good thermal conductor or has low reactivity reaction with water and acids. It is used in many heating applications because it doesnt corrode and has a high melting point.
Answer 1 of 10. Why is one metal better at conduction than the other one. All metals have some amount of resistivity to electrical currents which is why they require a power source to push the current through.
For this reason they are known as free electrons. Copper is also less oxidative than other metals. Any external influence will cause the repulsion action so the force of the.
Copper wire is used in power generation power transmission power distribution. It is used in many heating applications because it doesnt corrode and has a high melting point. What actually makes it a good conductor however is how some of its electrons are easily detached.
The need to increase. They are also known as conduction electrons because they help copper to be a good conductor of heat and electricity. This essentially forces the electricity down the piece of copper or conducts it down the metal.
The only other material that has similar resistance to. Many people have a general idea of what the word conductor means in an electrical context. The chain reaction is created by one electron moving.
This means that if you heat one end of a piece of copper the other end will quickly reach the same temperature. Most metals are pretty good conductors. Few reasons are considered for copper being a good conductor of electricity.
This is why copper wires are used in mains cables in houses and underground although overhead cables tend be aluminium because it is less dense. Each copper atom has lost one electron and become a positive ion. However apart from silver copper is the best.
Because Copper is an excellent electrical conductor most of its common uses are for electrical purposes. The only other material that has similar resistance to corrosion is stainless steel. To attribute to an aluminium conductor the same resistance as a copper conductor the cross-sectional area of the aluminium conductor must become larger to compensate for aluminiums higher.
Copper is a good conductor of electricity because of its huge charge density allowing more electrons to flow in its conducting system. Copper is a good conductor of heat. Silver is the best metallic conductor then comes copper.
Thick copper strip is used for lightning conductors on tall buildings like church spires. In a simple way. Answer 1 of 3.
The more free electrons in a metal the greater its conductivity. However apart from silver copper is the bestGood Thermal Conductivity. Copper has a significantly lower electrical volume resistivity.
However apart from silver copper is the best. One is coppers electrons Why are metals a good conductor of heat and Metals are a good conductor of heat and electricity because of their bonding. First and foremost copper is a good conductor because it is the least expensive of all conductors.
0017241 Ω x mm2m for copper compared to 00282 Ω x mm2m for aluminium This difference is highly relevant for power cables. Most metals are pretty good conductors. Metals are good conductors as the electrons in their outer shell are loose and can plunge out of the atom with the application of the slightest force voltage.
When the outer electrons detach it allows for a chain reaction of electrons. This means that if you heat one end of a piece of copper the other end will quickly reach the same temperature. If heat is applied to one end of a cooper wire it travels quickly to the other end as the electrons move unimpeded by resistance.
What determines the difference in conduction between 2 metals. However where size rather than weight is important copper is the best choice. A term conductor is defined as the very low energy easily required for a free electron to eject from valence energy level to the conduction levelin case of copper the.
Most metals are pretty good conductors. The cross sectional area of an aluminium conductor must be 56 larger than copper for the same current carrying capability. Copper is a good conductor for two reasons.
In this video I explain how conductors work on the atomic level. The lower the level of resistivity the more electrical conductivity a metal has. Copper has been used in electrical wiring since the invention of the electromagnet and the telegraph in the 1820s.
Copper is the electrical conductor in many categories of electrical wiring. It is the degree of energy within the metal that imparts heat. Most metals are pretty good conductors.
Since heat increases when the subatomic particles are able to move without resistance throughout the metal copper is a good conductor of heat. What makes a good conductor. Copper is a good conductor of heat.

Raw Materials Used In Copper Tube Copper Tubing Copper Raw Materials

This Picture Represents Really Well What A Conductor And A Insulator Are The Copper Is A Conductor Where The Electric Current Can Flow Freely Through It Meta

Mineral Ore From Rocks Minerals Eyewitness Minerals Rocks And Minerals Rock

Copper Coated Steel Wire Commonly Abbreviated As Ccs Is An Electrical Conductor Composed Cl Electrical Conductor Plastic Manufacturers Leather Office Supplies

Which Metals Have High Electrical Conductivity Electricity Conductive Materials Electrical Efficiency
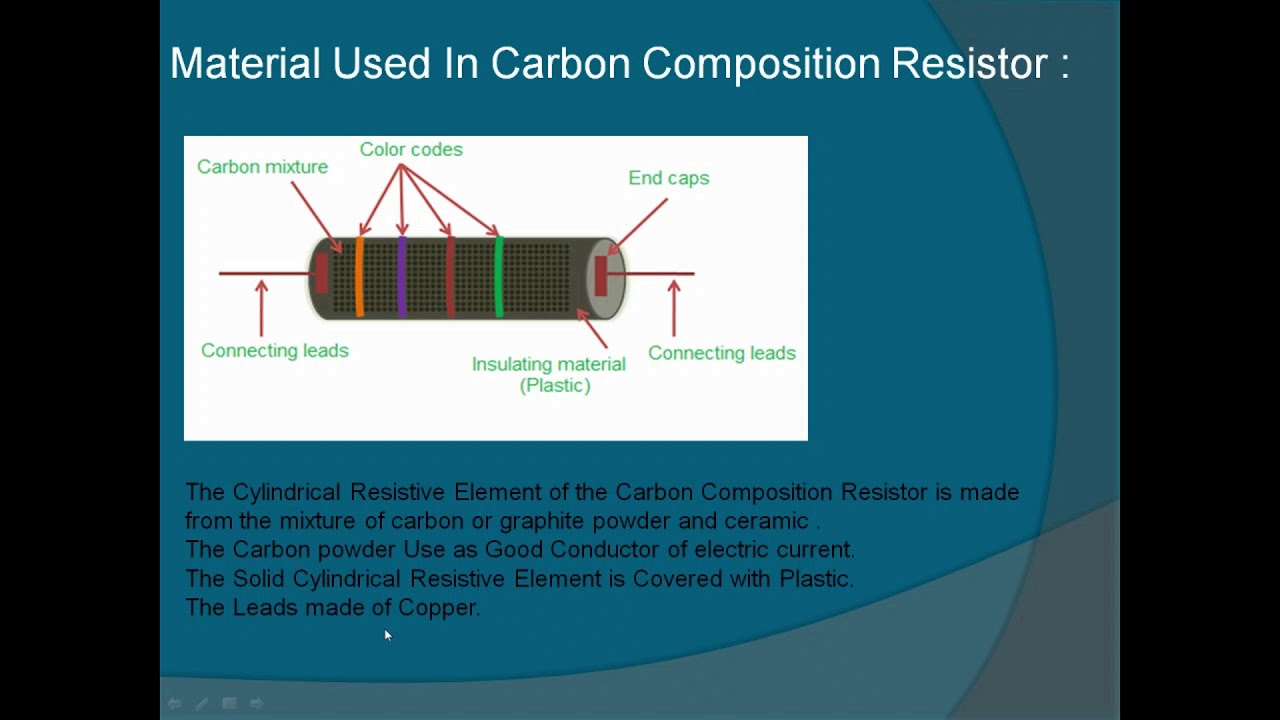
Https Youtu Be Lcqp1iqu1di Education Composition Resistor

Factors Physical Quantities Which Affect Resistance R Physics Physics Classroom Learn Physics

How To Tell The Impedance Of A Speaker A Guide For Everyone In 2021 Speaker Electronics Basics Tweeter Speaker

I Think This Photo It Best Represents What An Insulator And A Conductor Is Because The Wire Has Two Parts The Copper That It Allow To Pass The Electricity And

The Importance Of Good And Bad Conductors Of Heat Science Online Online Science Conductors Physical Science

A Quick Look At Different Types Of Multi Conductor Cables Copper Electrical Wire Manufacturing Conductors

Magikal Properties Of Copper Crystals Healing Properties Crystal Healing Stones Crystals And Gemstones

Pdf Print Complete Physics For Cambridge Igcse Cambridge Igcse Physics Physics Books

Metal Easy Science Science Flashcards Easy Science Chemical Changes

Copper Wire Manufacturing Project Report 2021 Plant Cost Manufacturing Process Industry Trends In 2021 Copper Wire Plant Projects Manufacturing Plant

The Electricity Teaching Pack Teaching Science Electricity Teaching Packs

No Title Science Notebooks Fourth Grade Science Science Lessons

10 Examples Of Electrical Conductors And Insulators Electrical Conductor Insulators And Conductors Conductors

Comments
Post a Comment